FDA will review that analysis as it also investigates these cases. The Advisory Committee on Immunization Practices ACIP provides advice and guidance to the Director of the CDC regarding use of vaccines and related agents for control of vaccine-preventable diseases in the civilian population of the United States.
 Advisory Committee On Immunization Practices Acip Cdc
Advisory Committee On Immunization Practices Acip Cdc
This report compiles and summarizes all recommendations from CDCs Advisory Committee on Immunization Practices ACIP for use of meningococcal vaccines in the United States.
Cdc advisory committee on immunization practices. The CDC and FDA lifted their pause on use of Johnson Johnsons coronavirus vaccine and said theyll add a safety warning about blood clots. These summary minutes provide an overview of the meeting which was devoted solely to the topic of coronavirus disease 2019 COVID-19 vaccines. Recommendations of the Advisory Committee on Immunization Practices ACIP This report summarizes the epidemiology of human papillomavirus HPV and associated diseases describes the licensed HPV vaccines provides updated data from clinical trials and postlicensure safety studies and compiles recommendations.
CDCs Advisory Committee on Immunization Practices ACIP CDCs Advisory Committee on Immunization Practices ACIP is seeking qualified PAs for ACIP Membership. This meeting is open to the public. The Centers for Disease Control and Prevention CDC cannot attest to the accuracy of a non-federal website.
Recommendations made by the ACIP are reviewed by the CDC Director and if adopted are published as official CDCHHS recommendations in. This report compiles and summarizes all previously published recommendations from CDCs Advisory Committee on Immunization Practices ACIP regarding prevention and control of pertussis tetanus. As a comprehensive summary and update of previously published recommendations it replaces all previously published reports and policy notes.
The meeting will be. The Centers for Disease Control and Prevention CDC convened an emergency meeting of its Advisory Committee on Immunization Practices ACIP on November 23 2020. The Centers for Disease Control and Prevention CDC sets the US.
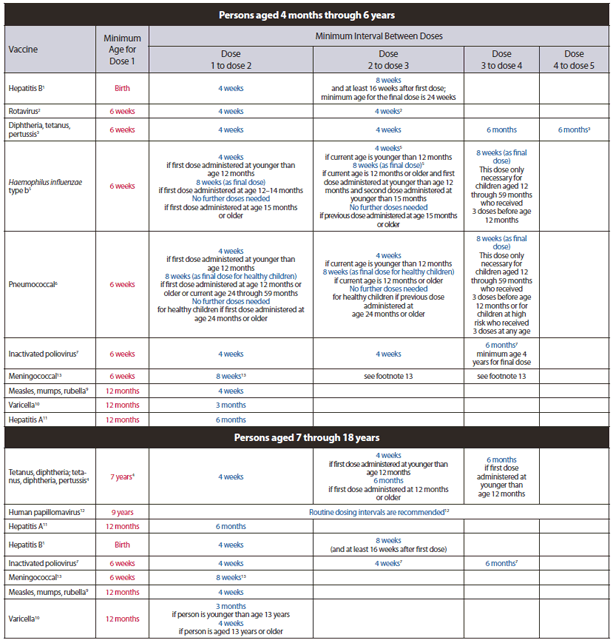
PDF - 18618 KB. Recommendation from the Advisory Committee on Immunization Practices ACIP for Use of Quadrivalent Meningococcal Conjugate Vaccine MCV4 in Children Aged 2--10 Years at Increased Risk for Invasive Meningococcal Disease Source. Adult and childhood immunization schedules based on recommendations from the Advisory Committee on Immunization Practices ACIP.
ACIP members are acknowledged experts with an outstanding record of achievement in their own field and an understanding of the immunization issues covered by ACIP. Links with this icon indicate that you are leaving the CDC website. MMWR December 7 2007.
Concurrent administration of antimicrobial agents and vaccines administration of live vaccines and Tuberculin Skin Tests TSTs and Interferon-gamma Release Assays IGRAs vaccination of preterm infants breastfeeding and vaccination vaccination during pregnancy persons vaccinated outside the United States vaccinating persons with increased bleeding risk. CDC reports recommendations from the Advisory Committee on Immunization Practices ACIP regarding prevention and control of tetanus diphtheria and pertussis in the United States. CDCs independent Advisory Committee on Immunization Practices met today to discuss the latest data on TTS hearing from the vaccine manufacturer Janssen and the COVID-19 Vaccine Safety.
The Advisory Committee on Immunization Practices shall provide advice and guidance to the Secretary HHS the Assistant Secretary for Health and the Director CDC regarding the most appropriate selection of vaccines and related agents for effective control of vaccine-preventable diseases in. In accordance with the Federal Advisory Committee Act the Centers for Disease Control and Prevention CDC announces the following meeting of the Advisory Committee on Immunization Practices ACIP. Before recommending any vaccine ACIP considers many factors including the safety and effectiveness of the vaccine.
CDC will convene an emergency meeting of the Advisory Committee on Immunization Practices ACIP on Wednesday April 14 2021 to further review these cases and assess potential implications on vaccine policy. The Centers for Disease Control and Prevention CDC convened an emergency meeting of its Advisory Committee on Immunization Practices ACIP on Decem. Advisory Committee on Immunization Practices.