The reported side effects from the vaccines include migraines anaphylaxis seizures paralysis and sudden death. These were most often reported one or two days after the day of vaccination and typically only.
 Public Needs To Prep For Vaccine Side Effects Science
Public Needs To Prep For Vaccine Side Effects Science
The mRNA vaccines are dependent upon reactogenicity which are the bodys transient but intense side effects after administering the vaccine.
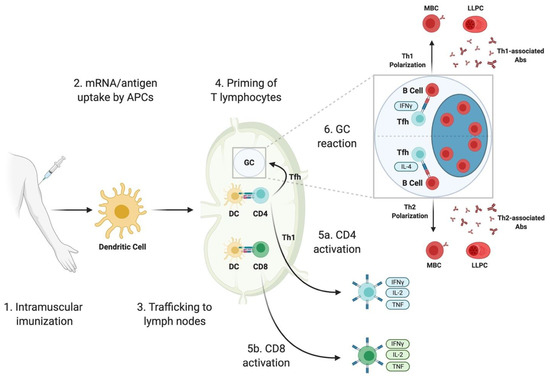
Mrna vaccine long term side effects. Anyone with a professional background in science or an interest in science knows that this is not the case at all. MRNA vaccines brought to market for human patientsIn order to receive Food and Drug Administration approval the companies will have to prove there are no immediate or short-term negative health effects from taking the vaccines. These are supposedly not long-term issues.
Serious reactions are uncommon and include rare cases of severe allergic reactions. MMR vaccine About 1 of 30000 recipients of measles mumps and rubella MMR vaccine can experience a temporary decrease in platelets. Could mRNA COVID-19 vaccines be dangerous in the long-term.
The most common side effect of the vaccine is painsoreness at injection site. Other common side-effects included fatigue headache muscle pain chills joint pain and fever. There is no explanation of any long-term side effects of taking this treatment.
But when the world begins inoculating itself with. Anyone whos over 70 who gets one of these COVID-19 mRNA vaccines will probably be sadly die within about two to three years. In order to receive Food and Drug Administration approval the companies will have to prove there are no immediate or short-term negative health effects from taking the vaccines.
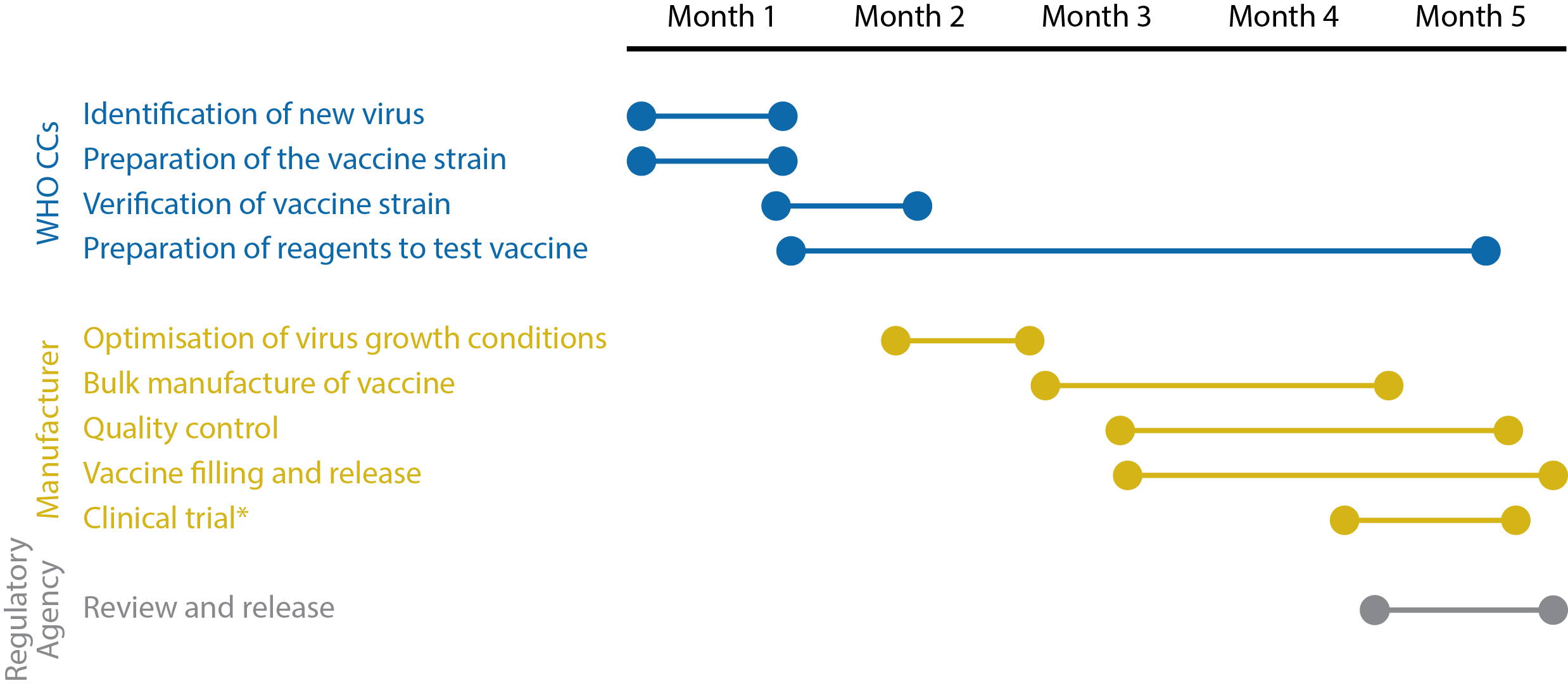
First most negative effects occur within 6 weeks of receiving a vaccine which is why the FDA asked the companies to provide 8 weeks of safety data. But thats just the mRNA. The only evidence on safety of mRNA vaccines comes from small phase I and phase II trials of SARS-CoV-2 vaccines with follow-up typically less than two months.
Platelets are the cells responsible for clotting of blood. The New Orleans. Systemic adverse events such as fatigue muscle aches headache and chills are common.
According to the Childrens Hospital of Philadelphia the vaccine is not expected to have long-term negative effects for a few reasons. Experts believe long-term effects from the gene therapy may include prion diseases such as Alzheimers cancers kidney diseases and microvascular injuries to the brain liver and heart. They have told the world that this does not interfere with your DNA.
Of course the only way to know what if any long-term side effects result from the use of these mRNA vaccines is to follow the participants of the Pfizer and Moderna clinical trials vaccinate. And since the mRNA vaccine does not introduce a virus inside of your body There should be no concern that the side effects are a sign that the vaccine is giving you the infection but rather. These side effects include immediate allergic reactions and a severe type of allergic reaction called anaphylaxis.
Decades of studying mRNA have shown no long-term side-effects. The average onset of symptoms occurred within seven weeks of vaccination. However theyre quite severe especially after the second dose of the vaccine series proposed for the mRNA vaccines for COVID-19.
Please try again later. Another worry is the potential for mRNA vaccines to be maliciously deployed to trick a persons body into attacking critical functions like cell repair fertility and neurological function. Immediate allergic reactions typically occur within 4 hours of receiving the.
So common side effects of mRNA vaccines such as fatigue myalgia or headache only last for 12 days. A condition called thrombocytopenia. Other commonly reported side effects include fever fatigue muscle aches or headache but these are usually mild and resolve in a few days.






:max_bytes(150000):strip_icc()/how-long-are-rabies-shots-good-3385625__FINAL-5bedb5ba46e0fb00518ad5fe.png)













/770535-article-why-did-i-get-sick-after-a-flu-shot-01-5a72326a3418c6003620c85d.png)





