In a Phase I Clinical trial that began in 2018 scientists at the International AIDS Vaccine Initiative IAVI and Scripps Research gave 48 participants two doses of either the vaccine candidate or. The targeted response was detected in 97.
 Hiv Vaccine Trial Participation In 2019 Avac
Hiv Vaccine Trial Participation In 2019 Avac
A recombinant Adenovirus-5 HIV vaccine called V520 has been tested in two Phase 2b studies Phambili and STEP.

Hiv vaccine trial. Large scale randomized control trials have shown these vaccines are safe and highly effective at preventing severe COVID-19. On December 13 2004 they began recruiting for the STEP study a 3000-participant phase II clinical trialof a novel HIV vaccine at sites in North America South America the Caribbean and Australia. The experimental vaccine developed by the International AIDS Vaccine Initiative IAVI and Scripps Research displayed a 97 response rate among participants in an early stage Phase I trial according to ABC News.
First-in-human clinical trial confirms new HIV vaccine approach. 1987 The first HIV vaccine clinical trial opened at the National Institutes of Health NIH Clinical Center in Bethesda Maryland. Longer duration studies and more data on people with underlying conditions including people with HIV PWH are awaited.
The trial called HPX3002HVTN 706 or Mosaico will assess whether an investigational vaccine regimen designed to induce immune responses against a variety of global HIV. HIV is a lifelong infection that attacks the immune. The vaccine showed success in stimulating production of rare immune cells needed to start the process of generating antibodies against the fast-mutating virus.
The HIV Vaccine Trials Network an international collaboration focused on the creation of an HIV vaccine currently has two other ongoing vaccine trials. Complementary study in women is ongoing. These are very early studies.
A phase 1 clinical trial confirms that the first stage of a novel approach to vaccination is safe and could in principle work against HIV. NEW YORK and LA JOLLA CA A phase 1 clinical trial testing a novel vaccine approach to prevent HIV has produced promising results IAVI and Scripps Research announced today. RV 144 or the Thai trial is the name of an HIV vaccine clinical trial combining two vaccines that failed on their own vaccinating in Thailand over the course of 24 weeks in October 2003 then testing for HIV until July 2006 publicly releasing efficacy findings in September 2009.
Although available data are currently insufficient to allow in-depth analyses specific to PWH the. The experimental vaccine primed the immune system as the first stage in the production of broadly neutralizing antibodies. Now preliminary data from an early stage clinical trial out of the International AIDS Vaccine Initiative and The Scripps Research Institute in La Jolla California suggests that a new HIV vaccine may hold promise.
Study will enroll men who have sex with men and transgender people. A novel vaccine approach for the prevention of HIV has shown promise in Phase I trials reported IAVI and Scripps Research. Also COVID by virtue of its structure and the way it attacks the body is a much easier virus to counteract.
This Phase 1 trial enrolled 138 healthy HIV-negative volunteers. Currently HIV affects more than 38 million people globally. A new HIV vaccine has shown a 97 response rate in Phase I clinical trials.
The strategy would involve a series of vaccinations that. The National Institutes of Health and partners today announced plans to conduct a Phase 3 HIV vaccine efficacy trial at multiple clinical research sites in North America South America and Europe. HIV was identified as the cause of AIDS.
The study sets the stage for additional clinical trials that will seek to refine and extend the approachwith the long-term goal of creating a safe and effective HIV vaccine. HHS Secretary Margaret Heckler declared that an AIDS vaccine will be ready for testing within two years. If approved this vaccine could become the first stage of a.
HVTN 705 and HVTN 706. They fought for it. But in studying HIV science advanced a great deal in all autoimmune diseases and its because of the advances made in the study of HIV that developing the vaccine for COVID advanced so quickly.
In another setback in the long quest to prevent HIV. Infection a trial in South Africa has been shut down because an experimental vaccine was. But being a part of the vaccine trials was not always an option for him or the 12 million HIV-positive people in the US.
The trial which started in 2018 was conducted with 48 HIV-negative adults and will serve as preliminary basis off which Moderna and its collaborators will further study and test a vaccine. According to the organisations the vaccine successfully stimulated the production of the rare immune cells needed to generate antibodies against HIV in.
 One Step Forward Two Steps Back Will There Ever Be An Aids Vaccine Nejm
One Step Forward Two Steps Back Will There Ever Be An Aids Vaccine Nejm
 New Hiv Vaccine Trial To Launch In U S Latin America And Europe
New Hiv Vaccine Trial To Launch In U S Latin America And Europe
Vaccines On Trial Hiv The Scientist Magazine
 Phase I Ii Clinical Trials Ongoing Scheduled Of Hiv Vaccines Left Download Scientific Diagram
Phase I Ii Clinical Trials Ongoing Scheduled Of Hiv Vaccines Left Download Scientific Diagram
 Results Of Truly Disappointing Hiv Vaccine Trial Published
Results Of Truly Disappointing Hiv Vaccine Trial Published
 Will We Ever Have A Hiv Vaccine Vaccinestoday
Will We Ever Have A Hiv Vaccine Vaccinestoday

 Hiv Vaccine In 2021 Leading Experts Optimistic About Ongoing Trials
Hiv Vaccine In 2021 Leading Experts Optimistic About Ongoing Trials
 Another Hiv Vaccine Clinical Trial Fails The Scientist Magazine
Another Hiv Vaccine Clinical Trial Fails The Scientist Magazine
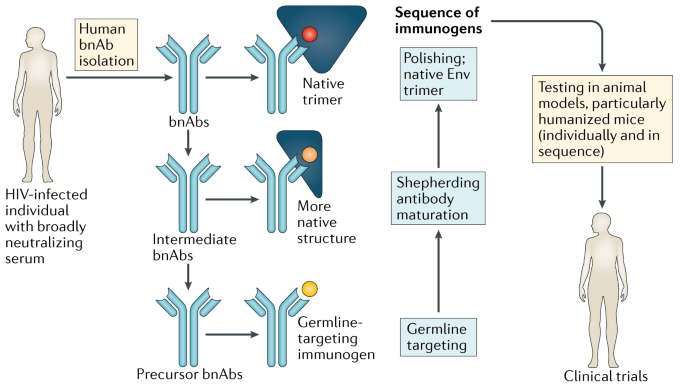 Advancing An Hiv Vaccine Advancing Vaccinology Nature Reviews Immunology
Advancing An Hiv Vaccine Advancing Vaccinology Nature Reviews Immunology
 Hiv Vaccine Clinical Trials Snapshot Avac
Hiv Vaccine Clinical Trials Snapshot Avac
 Novel Hiv Vaccine Approach Shows Promise In Landmark Trial
Novel Hiv Vaccine Approach Shows Promise In Landmark Trial


No comments:
Post a Comment
Note: Only a member of this blog may post a comment.