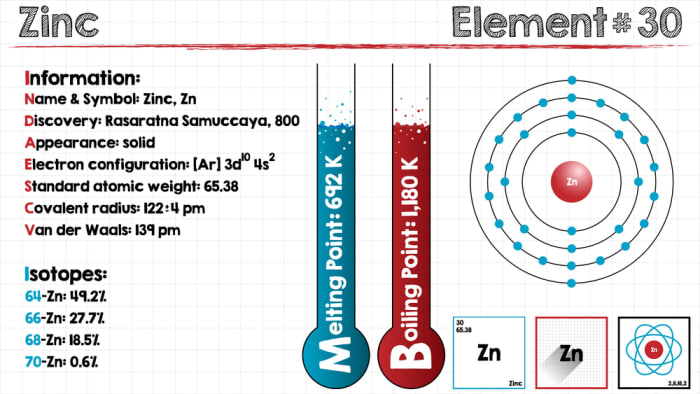
At room temperature zinc is brittle and blue-white in color but it can be polished to a bright finish. The electron configuration of the outermost shell of the atom is 4s².
With the chemical symbol Zn and the atomic number 30 zinc presents a bluish-white lustrous appearance.

Properties of zinc. For zincs mineralogical properties see native element. It may react both with acids and alkalis. The metal is brittle and less dense than iron but becomes malleable at a temperature between 100 and 150 o C.
Zinc is highly reactive with dilute acids causing the release of hydrogen. Zinc in the environment. Zinc metal is included in most single tablet it is believed to possess anti-oxidant properties which protect against premature aging of the skin and muscles of the body.
It is a fair electrical conductor and will react with dilute acids to release hydrogen. The zinc ends up in various proteins particularly in enzymes involved in the development of the body digestion and fertility. Zinc is an essential trace element in the human body where it is found in high concentration in the red blood cells as an essential part of the enzyme carbonic anhydrase which promotes many reactions relating to carbon dioxide metabolism.
Physical properties of Zinc. It has an atomic weight of 6538 gmol and an atomic number of 30. Zinc exists mostly in the sulfide state in.
The atomic number of zinc is 30 and is represented by the symbol Zn. Ad Experts in Trimix and we are standing by to help you now. Zinc is located in group IIB of the periodic table.
Zincs melting point is only 4195 o C and its boiling point is 907 o C. Physical zinc Properties. Zinc is a dense metal though less dense than iron meaning that it is heavy and will sink in water.
By its properties it is an amphoteric metal ie. The metal is blueish-white brittle at ambient temperatures and has a low boiling point and low melting point. When the temperature reaches 225C zinc oxidation is intense.
A shortage of zinc in the diet can lead to delayed healing skin irritation and loss of the sense of taste and encourages many chronic illnesses. The metal is lustrous but when the metal is seen in a commercial grade it has a dull finish to it. It belongs to the d-block period 4 and group 12 of the periodic table.
Zinc is a faily reactive metal that will combine with xygen and other non-metals. Its constant and only additional oxidation state is 2. Zinc metal tarnishes in mosit air and burns in air to form the white zinc oxide.
A base metal zinc is primarily used to galvanize steel a process that protects the metal against unwanted corrosion. The chemical properties that make up zinc include. Zinc has many physical properties.
Chemical properties of zinc As zinc is an element of the side subgroup we may say that it is inclined to form complexes it is the central atom of a complex particle. Zinc is a blue-white metal. Zinc is easily soluble in acid and it is also easy to replace gold silver copper etc.
Zinc is a bluish-white metal with a lustrous appearance. It is brittle by nature but exhibits good malleability and ductility at higher temperatures. At normal room temperature it remains brittle with a crystalline state.
Ad Experts in Trimix and we are standing by to help you now. One of the properties of zinc is that when heated between 110 o C to 150 o C it becomes malleable and ductile. Zinc is a very common substance that occurs naturally.
Some of the most astounding physical properties of zinc include. Many foodstuffs contain certain concentrations of zinc. Zinc Zn is an abundant metal found in the Earths crust with a myriad of industrial and biological uses.
 The Most Important Properties Of Zinc Oxide Property Value Download Table
The Most Important Properties Of Zinc Oxide Property Value Download Table
 Chemical And Physical Properties Of Zinc Mel Chemistry
Chemical And Physical Properties Of Zinc Mel Chemistry
 1 General Zinc Oxide Physical Properties Download Table
1 General Zinc Oxide Physical Properties Download Table
What Are The Physical Properties Of Zinc Mccnsulting Web Fc2 Com
 Comparison Of Physical And Mechanical Properties Of Zinc Alloys Click Image To Display Text Zinc Properties Of Zinc Mechanic
Comparison Of Physical And Mechanical Properties Of Zinc Alloys Click Image To Display Text Zinc Properties Of Zinc Mechanic
 Influence Of Surface Properties Of Zinc Oxide Nanoparticles On Their Cytotoxicity Sciencedirect
Influence Of Surface Properties Of Zinc Oxide Nanoparticles On Their Cytotoxicity Sciencedirect
 The Physical Properties Of Zinc Oxide Download Scientific Diagram
The Physical Properties Of Zinc Oxide Download Scientific Diagram
Calcium And Zinc Lessons Blendspace
 4 Vital Things About Zinc That You Should Know
4 Vital Things About Zinc That You Should Know
 Properties Of Zinc Ac41a Alloy Download Table
Properties Of Zinc Ac41a Alloy Download Table
 Zinc Properties Uses Facts Britannica
Zinc Properties Uses Facts Britannica
 Table 2 From Zinc Oxide Nanostructures Synthesis And Properties Semantic Scholar
Table 2 From Zinc Oxide Nanostructures Synthesis And Properties Semantic Scholar

No comments:
Post a Comment
Note: Only a member of this blog may post a comment.